|
|
Phase separation in borosilicate and alkali earth silicate glasses
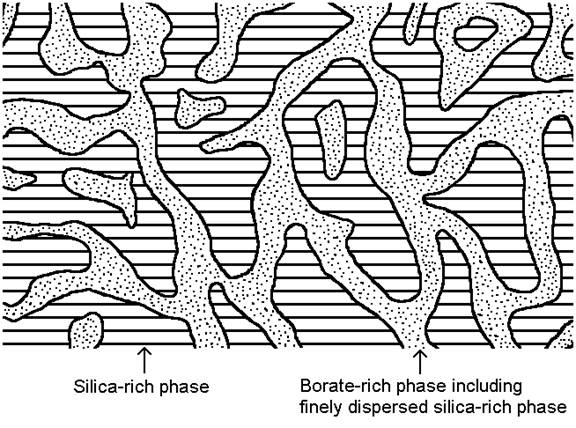
Phase separation in borosilicate and other glasses below the liquidus temperature has a significant influence on the properties [1, 2, 3]. Some theoretical background of phase separation is available online in reference [3]. The properties in phase separating glasses strongly depend on the thermal history, as the glass separates more or less into at least two phases. Borosilicate glasses in certain composition regions as given in the figures below tend to separate into a silica-rich phase, and a borate-rich phase upon heat treatment. In common industrial glass composition regions the silica-rich phase is continuous, while the borate-rich phase is either continuous given a sufficiently high borate concentration, or at low borate concentrations the borate-rich phase may be incorporated in the form of small droplets into the major silica-rich phase. Figure 1 shows the glass structure for the case that both, the silica-rich and borate-rich phases are continuous (interconnected) three-dimensionally throughout the whole sample.

Figure 1: Interconnected structure of phase-separated borosilicate glass
Often the borate-rich phase does contain finely dispersed droplets of the silica-rich phase due to the secondary phase separation effect [1, 2, 3] during cooling of the specimen.
Figure 2 displays the critical temperatures in the ternary system SiO2-B2O3-Na2O below which phase separation occurs. All concentrations in Figure 2 are in wt% and temperatures in oC. Figure 2 also shows the tie-lines at selected temperatures. The tie-lines give the chemical composition of the immiscible phases:
Figure 2: Metastable (sub-liquidus) phase separation in the system SiO2-B2O3-Na2O (click on the figure to enlarge)
The Figures 3 to 7 demonstrate a common phase separation trend in silicate and borosilicate glasses: The higher the ionic strength of the glass network modifying kation, the more pronounced phase separation occurs. Accordingly, the alkali and alkaline earth oxides promote phase separation in borosilicates in the following order: Cs2O (very little phase separation only below the liquidus temperature), Rb2O, K2O, Na2O, Li2O, BaO, SrO, CaO, MgO (very strong phase separation even above the liquidus temperature). The concentrations in Figures 3 to 7 are in mol% and temperatures in oC.
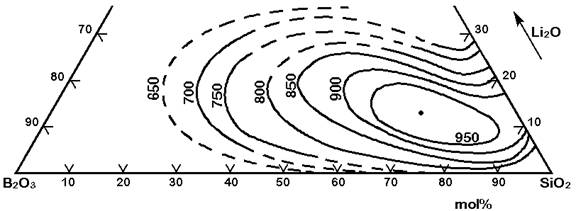
Figure 3: Metastable phase separation in the system SiO2-B2O3-Li2O, mol%
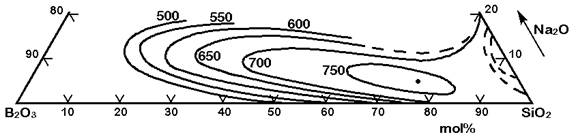
Figure 4: Metastable phase separation in the system SiO2-B2O3-Na2O, mol%
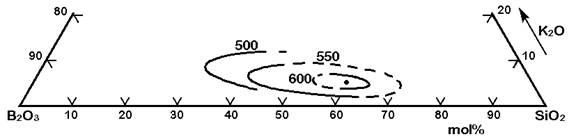
Figure 5: Metastable phase separation in the system SiO2-B2O3-K2O, mol%
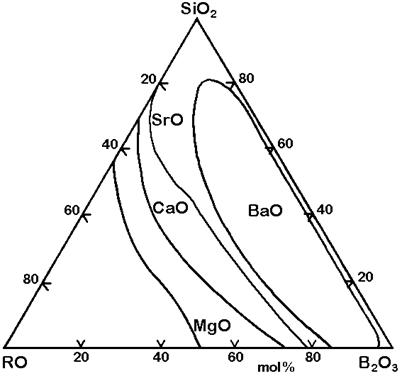
Figure 6: Stable phase separation in the systems SiO2-B2O3-RO, mol% [1]
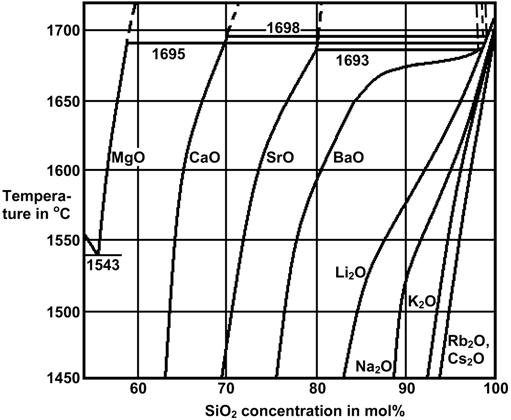
Figure 7: Stable and metastable phase separation in binary alkali and alkaline earth silicates [2]
During glass property modeling phase separation effects have to be considered, in the way that property values of phase separated glasses are excluded from the calculation. Only if a high number of experimental data are available concerning the influence of the thermal history on the properties of phase separating glasses, the thermal history may be considered as an additional independent variable for modeling.
References
[1] O. V. Mazurin: "Phase separation in glass", North-Holland, 1984
[2] Werner Vogel: "Chemistry of Glass"; The American Ceramic Society, 1985
[3] Online glass course by R. Brow: